lewis structure of h2s|H2S Lewis Structure, Molecular Geometry, Hybridization and : Baguio Learn how to draw the Lewis structure of H2S, a polar molecule with a bent shape and sp3 hybridization. Follow the steps to calculate valence electrons, identify the central atom, distribute electrons, check formal . As a former or retired City of Seattle employee, you have a period of 90 days where you have limited access to ESS. If you have never logged in as a former employee, please click on the 'Former Employee Registration' button below. If you have a former employee account, click on 'Former Employee Login' Former Employee Login Former Employee .
PH0 · Molecular Geometry, Lewis Structure, and Bond
PH1 · Lewis Structure of H2S, Hydrogen Sulfide
PH2 · Hydrogen Sulfide (H2S) Lewis Structure
PH3 · H2S Lewis structure
PH4 · H2S Lewis Structure: How to Draw the Dot Structure for H2S
PH5 · H2S Lewis Structure, Molecular Geometry, Hybridization and Polarity
PH6 · H2S Lewis Structure, Molecular Geometry, Hybridization and
PH7 · H2S Lewis Structure, Molecular Geometry
PH8 · H2S Lewis Structure, Geometry
PH9 · H2S Lewis Structure (Dihydrogen sulfide)
PH10 · H2S Lewis Structure
PH11 · H2S (Hydrogen sulfide) Lewis Structure
Author links open overlay panel Oscar Puig 1, Friederike Caspary 1, Guillaume Rigaut, Berthold Rutz, Emmanuelle Bouveret, Elisabeth Bragado-Nilsson, Matthias Wilm, Bertrand Séraphin 2 Show more Add to Mendeley
lewis structure of h2s*******A step-by-step explanation of how to write the Lewis Dot Structure for H2S (Dihydrogen Sulfide).The H2S Lewis structure is similar to the structure for water.
Learn how to draw the Lewis structure of H2S with eight valence electrons, and how it has sp3 hybridization, bent molecular geometry, and is nonpolar. Fin.
Learn how to draw the lewis structure of H2S, a covalent compound with no hybridization, and its molecular geometry, electron geometry, and molecular orbital diagram. Find out the conditions for .Learn how to draw the Lewis structure of H2S, a polar molecule with a bent shape and sp3 hybridization. Follow the steps to calculate valence electrons, identify the central atom, distribute electrons, check formal .Learn how to draw the lewis structure of H2S with two S-H bonds and two lone pairs on sulfur atom. Follow the steps of finding total valence electrons, center atom, lone pairs . Hydrogen and sulfur are both non-metals, and so they SHARE electrons to form covalent bonds (this makes it a covalent aka MOLECULAR compound). Sulfur .
Learn how to draw the Lewis structure of H2S, a molecule with two hydrogen atoms and one sulfur atom. Find out the number of valence electrons, the polarity, and .Learn how to draw the Lewis structure of hydrogen sulfide (H2S) using the octet rule and VSEPR theory. Find out the molecular geometry and bond angle of H2S with examples and references.H2S Lewis Structure, Molecular Geometry, Hybridization and Let's do the Lewis structure for H2S: Dihydrogen Sulfide. On the periodic table: Hydrogen, group 1, has 1 valence electron, but we have two Hydrogens here so let's multiply that .Learn how to draw the H2S Lewis structure, which shows the arrangement of atoms and electrons in the molecule. Find out the molecular geometry, hybridization, and electron geometry of H2S, and how they relate to its .
B. Steps in drawing the H2S Lewis structure. Here are the step-by-step instructions on how to draw the H2S Lewis structure: Determine the number of valence electrons: Find the total number of valence electrons .
Steps of drawing H2S lewis structure Step 1: Find the total valence electrons in H2S molecule. In order to find the total valence electrons in H2S molecule, first of all you should know the valence .lewis structure of h2s H2S Lewis Structure, Molecular Geometry, Hybridization and The Lewis structure of hydrogen sulfide is best represented as a bent H{eq}_2 {/eq}S molecule with two lone pairs of electrons on the S atom represented by two pairs of dots (or two bars). .Lewis dot structure represents the valence electrons participating in the bond formation and the nonbonding electrons remaining as lone pairs on the atoms. Dashes in Lewis structure represent bonds, and dots represent lone pairs. The structure is constructed using the octet rule [1-4]. Learn how to draw the Lewis dot structure of Hydrogen sulphide molecule through a step-by-step tutorial.
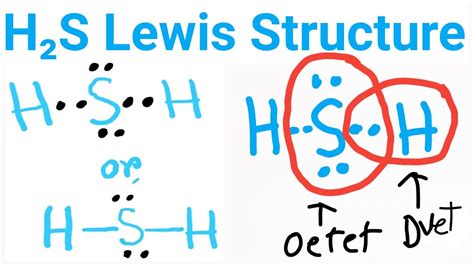
Steps. By using the following steps, you can easily draw the Lewis structure of H 2 S: #1 Draw skeleton #2 Show chemical bond #3 Mark lone pairs #4 Calculate formal charge and check stability (if octet is already completed on central atom). Let’s one by one discuss each step in detail.
Drawing the Lewis Structure for H 2 S. Viewing Notes: The Lewis structure for H 2 S is very similar to H 2 O. . Let's do the Lewis structure for H2S: Dihydrogen Sulfide. On the periodic table: Hydrogen, group 1, has 1 valence electron, but we have two Hydrogens here so let's multiply that by 2. Plus Sulfur is in group 6 or 16 on the periodic . The Lewis structure of H2S consists of a central sulphur atom (S) and two external hydrogen atoms (H) at a 92.1° degree bond angle. The sulphur atom (S) and the two hydrogen atoms (H) are each connected by a single bond. The Lewis structure of H2S is shown below:
lewis structure of h2sGet the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha. H2s lewis structure angle. In the H2S lewis structure, the intermixing 3s, 3p orbital form sp3 hybridized orbital, so the covalent bond angle should be 109.5̊ but it is lowered to 92.1̊ by the steric repulsion between dense two non bonding electron pair of ‘S’.
If you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Draw out a correct Lewis Structure for the following compounds. HCN; LiF; C 3 H 6 (two possibilities) CO 3 2-CH 3 NO 2; Answers: 1. 2. 3. or . 4. 5. Another simple and general procedure to draw Lewis structures has been proposed by A.B.P. Lever (see reference 5). Before beginning this procedure it is necessary to know the basic geometry . To know specifically about the electronic structure of H2S, you must also read an article on H2S lewis structure, geometry, hybridization. FAQs. Q1. Name the compounds that have a polar bond. .
What is the Lewis structure of [//substance:H2S//]? Natural Language; Math Input; Extended Keyboard Examples Upload Random. Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history, geography, engineering, .

Lewis structure of H2S||Draw the lewis dot structure of H2S ||lewis structure of H2SHow to draw the Lewis Structure for H2S ||Lewis structure of H2S step by.
The Lewis dot structure of hydrogen sulfide (H 2 S) consists of a sulfur (S) atom at the center. It is surrounded by 2 hydrogen (H) atoms, one on each side, by a single covalent bond. The central S-atom carries 2 lone pairs of electrons, while there is no lone pair on any of the terminal H-atoms.
A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below .
Learn the steps to draw the Lewis Structure of H2S (dihydrogen sulfide) in just 1 minute.📌You can draw any lewis structures by following the simple steps me.
ROYAL CANIN® Friends; Tilbage til toppen. Kontakt Royal Canin (+45)89153535; Kontakt os .
lewis structure of h2s|H2S Lewis Structure, Molecular Geometry, Hybridization and